You received
new message.
new messages.
Read now.
You received
new message.
new messages.
Read now.
Discover social connections
Find out what your friends are posting on LesPAC
With over 2.7 million LesPAC members, chances are that someone you know is advertising on LesPAC. Social connections help you find ads on LesPAC posted by your Facebook friends.
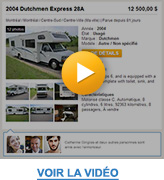
Imagine you're looking for a recreational vehicle. Social connections would allow you to see that the advertiser of the recreational vehicle that interests you on LesPAC is known by three of your "Facebook friends": Mathieu, Hugo, and Catherine. That increases your trust, doesn't it?
To find out what your friends are saying, log in to LesPAC via the Facebook button.
Privacy and freedom of choice!
Privacy is LesPAC's priority: Your personal information is protected. Social connections appear only to those who are part of your Facebook social network.
Enable or disable this feature on your My Profile page.